| From left to right: Katie Diebold, Anya Watson, the author Dana Sackett and Fernando Fuentes tracking fish (credit: Kenneth W. Able) |
several months over three years tracking the movements of summer flounder to better understand their habitat use and migration dynamics. While this method of tracking fish was and still is innovative, there are numerous other methods (e.g. tag-recapture, PIT tags, satellite tracking, side-scan sonar, DIDSON sonogram) that can also be used to answer questions about fish movements and their ecology. Scientists in Alaska have even started using mobile gliders to help track highly mobile ocean species with acoustic tags.
 |
| Glider used by Alaskan scientist to track fish (sciencedaily.com) |
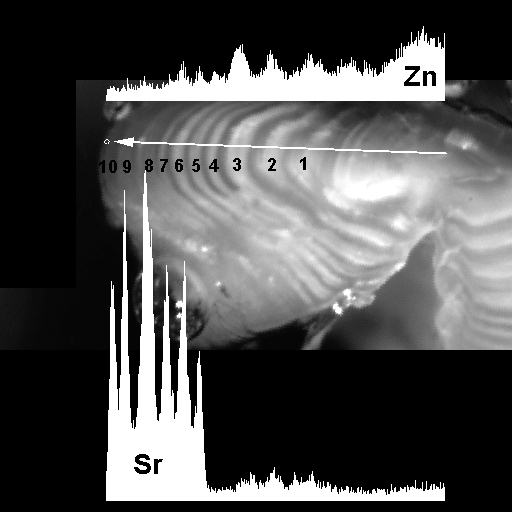 |
| Aged otolith with concentrations of Sr and Zn (home.cc.umanitoba.ca/~halden) |
A study by Godbout and others (2010) found that sulfur isotope ratios in tissue and otoliths were able to distinguish between anadromous and non-anadromous ecotypes of sockeye salmon. Strontium-calcium ratios in fish otoliths have also helped differentiate between freshwater, brackish and ocean contingents of salmon, striped bass and others. Further, analyzing trace elements on specific otolith annuli and comparing them to concentrations of those elements in water can provide a record of a fish’s movements over its life history.
 |
| Strontium maps and line transects of Sr:Ca ratios along the length of otoliths of three Japanese eels with habitats: a dark blue = fresh water, light blue = low salinities, and yellow and orange = higher salinities (Aoyama 2009). |
Nitrogen isotope ratios are most notably useful because there is a constant enrichment (~3.4‰) of the heavier nitrogen isotope with each trophic level allowing scientist to use this metric to determine the relative trophic position of an individual fish. However, to apply this approach one must also know the isotope ratio of nitrogen available to primary producers at the base of the food web; an easy thing to measure if fish are constrained to a lake. Without this information fish that fall within different food webs, with different nitrogen sources at the base, are difficult to compare.
In marine systems this would seem to be a problem because of the open boundaries and highly mobile nature of oceanic species, which makes identifying the base of the food web difficult. However, specific amino acids (i.e. phenylalanine) in fish tissue retain the nitrogen isotope signature at the base of the food web and can be used to rectify this difficulty. Contrary to nitrogen isotopes, carbon isotope ratios do not vary with trophic level; instead they are used to indicate whether the carbon in fish tissue originated from inshore versus offshore, or benthic versus pelagic sources.
Isotopes of other heavier elements such as mercury are also proving very useful in contaminant studies. A study by Senn and others (2010) used nitrogen, carbon and mercury isotopes to find that coastal and migratory oceanic species in the Gulf of Mexico are part of distinctly different food webs and derive the mercury in their tissues from different sources.
There are advantages and disadvantages to each of the methods discussed above. However, combining some of these measures could help maximize those advantages and provide a vast amount of information on fish ecology, as well as how contaminants are accumulating in fishes.
If you have any information about different tools used to understand fish ecology or can discuss the benefits or disadvantages of the methods mentioned here please share your experience or thoughts below.
Dana
References:
Aoyama J. 2009. Life History and Evolution of Migration in Catadromous Eels (Anguilla). ABSM. 2:1–42.
Godbout L, Trudel M, Irvine JR, Wood CC, Grove MJ, Schmitt AK, McKeegan KD. 2010. Sulfur isotopes in otoliths allow discrimination of anadromous and non-anadromous ecotypes of sockeye salmon (Oncorhynchus nerka). Environ Biol Fish 89:521-532.
Senn DB, Chesney EJ, Blum JD, Bank MS, Maage A, Shine JP. 2010. Stable isotope (N, C, Hg) study of methylmercury sources and trophic transfer in the northern Gulf of Mexico. Environ Sci Technol 44:1630-1637.
http://www.sciencedaily.com/releases/2010/06/100622095114.htm


No comments:
Post a Comment
Please leave a professional comment to create discussion about this topic. We reserve the right to remove any comment.